How Fast Does Heat Travel
Throughout the universe, it'southward natural for energy to menstruum from one place to some other. And unless people interfere, thermal energy — or rut — naturally flows in one direction only: from hot toward cold.
Heat moves naturally past any of iii ways. The processes are known as conduction, convection and radiation. Sometimes more than than one may occur at the aforementioned fourth dimension.
First, a picayune groundwork. All matter is fabricated from atoms — either single ones or those bonded in groups known as molecules. These atoms and molecules are ever in movement. If they have the same mass, hot atoms and molecules motion, on average, faster than cold ones. Even if atoms are locked in a solid, they still vibrate back and forth around some average position.
In a liquid, atoms and molecules are free to flow from place to place. Within a gas, they are even more free to move and will completely spread out within the volume in which they are trapped.
Some of the virtually easily understood examples of oestrus flow occur in your kitchen.
Conduction
Put a pan on a stovetop and turn on the estrus. The metal sitting over the burner will be the first office of the pan to go hot. Atoms in the pan's bottom will start to vibrate faster every bit they warm. They also vibrate further dorsum and along from their average position. Every bit they bump into their neighbors, they share with that neighbor some of their energy. (Call up of this as a very tiny version of a cue brawl slamming into other balls during a game of billiards. The target balls, previously sitting still, gain some of the cue ball'due south energy and motility.)
As a result of collisions with their warmer neighbors, atoms commencement moving faster. In other words, they are now warming. These atoms, in plow, transfer some of their increased energy to neighbors even farther from the original source of heat. This conduction of heat through a solid metal is how the handle of a pan gets hot even though it may be nowhere near the source of heat.
Convection
Convection occurs when a material is gratis to move, such as a liquid or a gas. Once again, consider a pan on the stove. Put water in the pan, then plough on the heat. Every bit the pan gets hot, some of that heat transfers to the molecules of water sitting on the bottom of the pan via conduction. That speeds up the motion of those water molecules — they are warming.

As the water warms, it now begins to expand. That makes it less dense. It rises above denser water, carrying abroad heat from the bottom of the pan. Cooler water flows down to take its identify next to the hot bottom of the pan. Every bit this water warms, it expands and rises, ferrying its newly-gained free energy with it. In brusk order, a round period of rising warm water and falling cooler water sets upwardly. This circular blueprint of heat transfer is known as convection.
It's also what largely warms food in an oven. Air that's warmed by a heating element or gas flames at the summit or bottom of the oven carries that rut to the fundamental zone where the nutrient sits.
Air that's warmed at Earth'south surface expands and rises merely similar the water in the pan on the stove. Large birds such equally frigate birds (and human flyers riding engineless gliders) often ride these thermals — rising blobs of air — to gain distance without using whatever energy of their own. In the ocean, convection caused by heating and cooling helps to drive ocean currents. These currents move water around the globe.
Radiation
The third type of energy transfer is in some ways the about unusual. It can move through materials — or in the absenteeism of them. This is radiations.
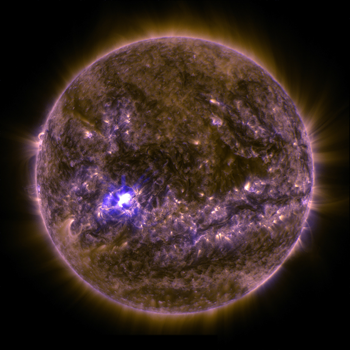
Consider visible lite, a form of radiation. It passes through some types of glass and plastic. X-rays, another grade of radiation, readily pass through flesh but are largely blocked by os. Radio waves pass through the walls of your home to reach the antenna on your stereo. Infrared radiation, or heat, passes through the air from fireplaces and light bulbs. Simply unlike conduction and convection, radiation doesn't require a material to transfer its energy. Low-cal, X-rays, infrared waves and radio waves all travel to Earth from the far reaches of the universe. Those forms of radiation will laissez passer through plenty of empty space along the manner.
10-rays, visible light, infrared radiations, radio waves are all different forms of electromagnetic radiation. Each blazon of radiation falls into a particular band of wavelengths. Those types differ in the amount of energy they have. In general, the longer the wavelength, the lower the frequency of a particular blazon of radiation and the less energy it will behave.
To complicate things, it's important to note that more than ane form of heat transfer may occur at the same time. A stove's burner not only heats a pan but as well the nearby air and makes it less dense. That carries warmth upward via convection. But the burner also radiates heat as infrared waves, making things nearby warm upwardly. And if you're using a cast-atomic number 26 skillet to cook a tasty meal, be sure to grab the handle with a potholder: It'south gonna be hot, thanks to conduction!
How Fast Does Heat Travel,
Source: https://www.snexplores.org/article/explainer-how-heat-moves
Posted by: wilsonmeself.blogspot.com


0 Response to "How Fast Does Heat Travel"
Post a Comment