Ba On The Periodic Table
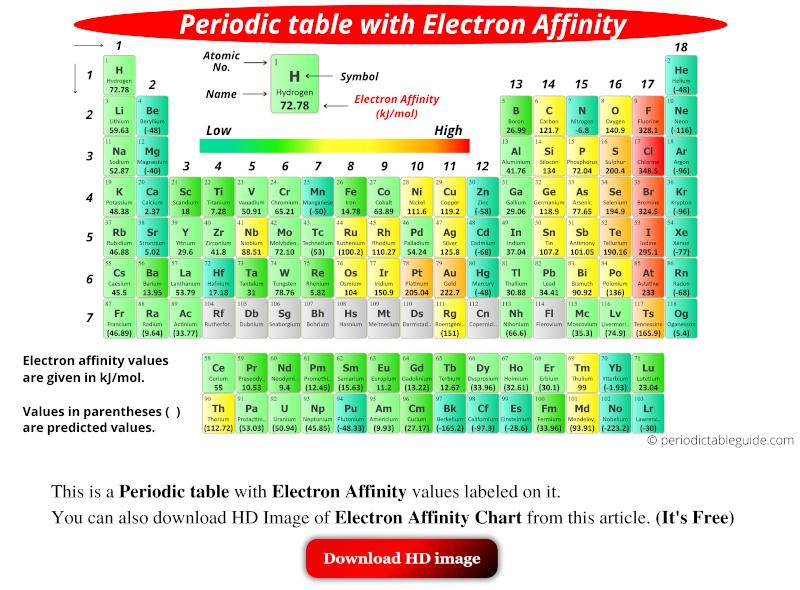
Periodic table with electron affinity values is shown higher up. The values of electron analogousness are given in kJ/mol. Values in parentheses ( ) are predicted values.
Electron affinity is the amount of free energy change (ΔE) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
In other words, when the electron is added to a neutral cantlet, the free energy is either released or absorbed. And this amount of energy modify (ΔE) is called electron affinity.
This free energy change (ΔE) tin be positive, negative or zero.
And the sign of Electron Affinity (EEA) is opposite to the sign of energy change (ΔE).
Eastward EA = – (ΔE)
In simple words, if ΔE is positive, so Due eastEA will be negative. And if ΔE is negative, and then EEA will be positive.
Points to remember:
- For exothermic reaction (i.e when energy is released), the modify in energy (ΔE) is negative. Hence the sign of Electron Analogousness (E EA ) volition be positive.
- For endothermic reaction (i.due east when energy is absorbed), the modify in energy (ΔE) is positive. Hence the sign of Electron Affinity (Eastward EA ) volition be negative.
- For neutral process (i.due east when energy is neither absorbed nor released), the value of Electron Affinity (EastEA) volition be zero.
More the positive values of Electron Affinity (EEA), more is the free energy released.
Halogens on the periodic tabular array shows more positive values of Electron Analogousness (EastEA). Hence they indicate that more energy is released when an electron is added to the neutral atom.
Electron Affinity Values of All Elements
| Diminutive number | Elements | Electron affinity (kJ/mol) |
|---|---|---|
| 1 | Hydrogen (H) | 72.78 |
| 2 | Helium (He) | (-48) |
| 3 | Lithium (Li) | 59.63 |
| 4 | Beryllium (Be) | (-48) |
| 5 | Boron (B) | 26.99 |
| vi | Carbon (C) | 121.77 |
| 7 | Nitrogen (Northward) | -6.8 |
| 8 | Oxygen (O) | 140.98 |
| 9 | Fluorine (F) | 328.sixteen |
| 10 | Neon (Ne) | (-116) |
| xi | Sodium (Na) | 52.87 |
| 12 | Magnesium (Mg) | (-40) |
| 13 | Aluminum (Al) | 41.76 |
| 14 | Silicon (Si) | 134.06 |
| 15 | Phosphorus (P) | 72.04 |
| xvi | Sulfur (S) | 200.4 |
| 17 | Chlorine (Cl) | 348.57 |
| xviii | Argon (Ar) | (-96) |
| 19 | Potassium (K) | 48.38 |
| 20 | Calcium (Ca) | ii.37 |
| 21 | Scandium (Sc) | 18 |
| 22 | Titanium (Ti) | vii.28 |
| 23 | Vanadium (V) | l.91 |
| 24 | Chromium (Cr) | 65.21 |
| 25 | Manganese (Mn) | (-l) |
| 26 | Iron (Fe) | 14.78 |
| 27 | Cobalt (Co) | 63.89 |
| 28 | Nickel (Ni) | 111.65 |
| 29 | Copper (Cu) | 119.23 |
| 30 | Zinc (Zn) | (-58) |
| 31 | Gallium (Ga) | 29.06 |
| 32 | Germanium (Ge) | 118.93 |
| 33 | Arsenic (As) | 77.65 |
| 34 | Selenium (Se) | 194.95 |
| 35 | Bromine (Br) | 324.53 |
| 36 | Krypton (Kr) | (-96) |
| 37 | Rubidium (Rb) | 46.88 |
| 38 | Strontium (Sr) | v.02 |
| 39 | Yttrium (Y) | 29.six |
| forty | Zirconium (Zr) | 41.8 |
| 41 | Niobium (Nb) | 88.51 |
| 42 | Molybdenum (Mo) | 72.ten |
| 43 | Technetium (Tc) | (53) |
| 44 | Ruthenium (Ru) | (100.27) |
| 45 | Rhodium (Rh) | 110.27 |
| 46 | Palladium (Pd) | 54.24 |
| 47 | Silvery (Ag) | 125.86 |
| 48 | Cadmium (Cd) | (-68) |
| 49 | Indium (In) | 37.04 |
| l | Can (Sn) | 107.29 |
| 51 | Antimony (Sb) | 101.05 |
| 52 | Tellurium (Te) | 190.16 |
| 53 | Iodine (I) | 295.15 |
| 54 | Xenon (Xe) | (-77) |
| 55 | Cesium (Cs) | 45.5 |
| 56 | Barium (Ba) | 13.95 |
| 57 | Lanthanum (La) | 53.79 |
| 58 | Cerium (Ce) | 55 |
| 59 | Praseodymium (Pr) | ten.53 |
| 60 | Neodymium (Nd) | 9.4 |
| 61 | Promethium (Pm) | (12.45) |
| 62 | Samarium (Sm) | (xv.63) |
| 63 | Europium (European union) | 11.2 |
| 64 | Gadolinium (Gd) | (13.22) |
| 65 | Terbium (Tb) | 12.67 |
| 66 | Dysprosium (Dy) | (33.96) |
| 67 | Holmium (Ho) | (32.61) |
| 68 | Erbium (Er) | (30.ane) |
| 69 | Thulium (Tm) | 99 |
| lxx | Ytterbium (Yb) | (-1.93) |
| 71 | Lutetium (Lu) | 23.04 |
| 72 | Hafnium (Hf) | 17.eighteen |
| 73 | Tantalum (Ta) | 31 |
| 74 | Tungsten (W) | 78.76 |
| 75 | Rhenium (Re) | 5.82 |
| 76 | Osmium (Os) | 104 |
| 77 | Iridium (Ir) | 150.94 |
| 78 | Platinum (Pt) | 205.04 |
| 79 | Aureate (Au) | 222.75 |
| 80 | Mercury (Hg) | (-48) |
| 81 | Thallium (Tl) | 30.88 |
| 82 | Lead (Pb) | 34.41 |
| 83 | Bismuth (Bi) | xc.92 |
| 84 | Polonium (Po) | (136) |
| 85 | Astatine (At) | 233 |
| 86 | Radon (Rn) | (-68) |
| 87 | Francium (Fr) | (46.89) |
| 88 | Radium (Ra) | (9.64) |
| 89 | Actinium (Air-conditioning) | (33.77) |
| 90 | Thorium (Th) | (112.72) |
| 91 | Protactinium (Pa) | (53.03) |
| 92 | Uranium (U) | (50.94) |
| 93 | Neptunium (Np) | (45.85) |
| 94 | Plutonium (Pu) | (-48.33) |
| 95 | Americium (Am) | (ix.93) |
| 96 | Curium (Cm) | (27.17) |
| 97 | Berkelium (Bk) | (-165.24) |
| 98 | Californium (Cf) | (-97.31) |
| 99 | Einsteinium (Es) | (-28.vi) |
| 100 | Fermium (Fm) | (33.96) |
| 101 | Mendelevium (Md) | (93.91) |
| 102 | Nobelium (No) | (-223.22) |
| 103 | Lawrencium (Lr) | (-thirty.04) |
| 104 | Rutherfordium (Rf) | unknown |
| 105 | Dubnium (Db) | unknown |
| 106 | Seaborgium (Sg) | unknown |
| 107 | Bohrium (Bh) | unknown |
| 108 | Hassium (Hs) | unknown |
| 109 | Meitnerium (Mt) | unknown |
| 110 | Darmstadtium (Ds) | unknown |
| 111 | Roentgenium (Rg) | (151) |
| 112 | Copernicium (Cn) | unknown |
| 113 | Nihonium (Nh) | (66.vi) |
| 114 | Flerovium (Fl) | unknown |
| 115 | Moscovium (Mc) | (35.iii) |
| 116 | Livermorium (Lv) | (74.9) |
| 117 | Tennessine (Ts) | (165.9) |
| 118 | Oganesson (Og) | (five.403) |
Gratis Gift for yous: Interactive Periodic Table
Let me tell you how this Interactive Periodic Tabular array volition assistance yous in your studies.
i). You tin can effortlessly discover every single detail about the elements from this unmarried Interactive Periodic table.
2). You lot will go the detailed information about the periodic tabular array which will convert a newbie into pro.
3). Yous volition also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it's high resolution paradigm now (Information technology'south FREE)
External links:
Electron affinity of elements: Wikipedia
Ba On The Periodic Table,
Source: https://periodictableguide.com/electron-affinity-chart-periodic-table/
Posted by: wilsonmeself.blogspot.com


0 Response to "Ba On The Periodic Table"
Post a Comment